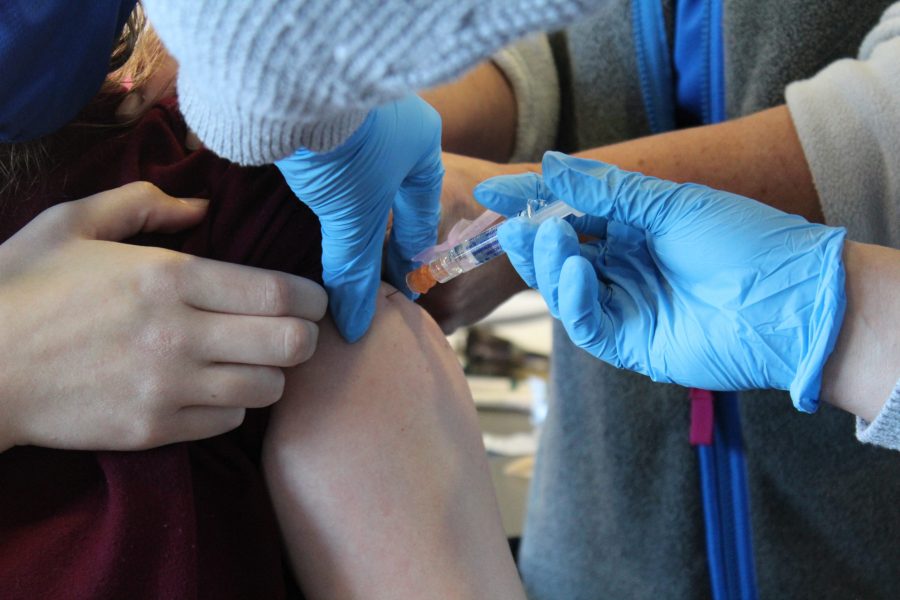
As of April 13, the Centers for Disease Control and Prevention and the Food and Drug Administration have called for the Johnson & Johnson vaccine, also known as Janssen, to halt in its distribution due to rare blood clots forming in some recipients. The CDC says, “People who have received the J&J/Janssen COVID-19 vaccine within the past three weeks who develop severe headache, abdominal pain, leg pain, or shortness of breath should seek medical care right away.”
These statements were made after six women between the ages of 18-48 experienced a rare blood clot called cerebral venous sinus thrombosis, or CVST. The FDA says that this type of clot is seen in people with low levels of blood platelets. They also said, “Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, an anticoagulant drug called heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous.” Since this clot is rare and difficult to treat, the FDA and CDC are calling for a pause in vaccine distribution so healthcare workers can become aware of this possibility and prepare.
According to CNN, almost 7 million Johnson & Johnson vaccines have been administered in the United States alone. The CDC explains that in its clinical trials the vaccine was 66.3% effective, showing the most side effects in individuals who were between the ages of 18-59. According to Fox 5 Atlanta, Georgia health officials were some of the first to pause the distribution of the vaccine after multiple people fell ill after their vaccinations at a Cumming site. They continue saying, “The pause in using the Johnson & Johnson vaccine will impact Atlanta Public Schools’ plan to get its educators inoculated.”
Ethan Pilcher, a former University of North Georgia student, is employed with the Hall County schools, and received his vaccine on April 11 through the school system. He explains that he got the vaccine because he has elderly family members, and is getting married in June where he hopes to travel overseas for the honeymoon. He says, “I was worried about the vaccine at first because of negative things that people would say. I ultimately chose to do it because I thought the pros outweigh the cons.” However, after learning about the pause in the Johnson & Johnson vaccine Pilcher says, “It caused me to worry, but once I found out that it was only six people out of almost 7 million I felt better.” The only symptoms Pilcher says that he and his fellow co-workers have experienced after the vaccine were headaches, fevers, and body aches for about a day. He agrees that the pause was the right choice saying, “I think that the CDC and FDA should investigate the issue even though it was only six that number can grow if it is still being administered without figuring out the problem.”
Johnson & Johnson’s Vice Chairman of the Executive Committee and Chief Scientific Officer, Paul Stoffels, came out with a statement after the company paused the roll out saying, “We continue to believe in the positive benefit-risk profile of our vaccine. We value the consideration of the Advisory Committee, and we will continue to collaborate with medical experts and global health authorities.”